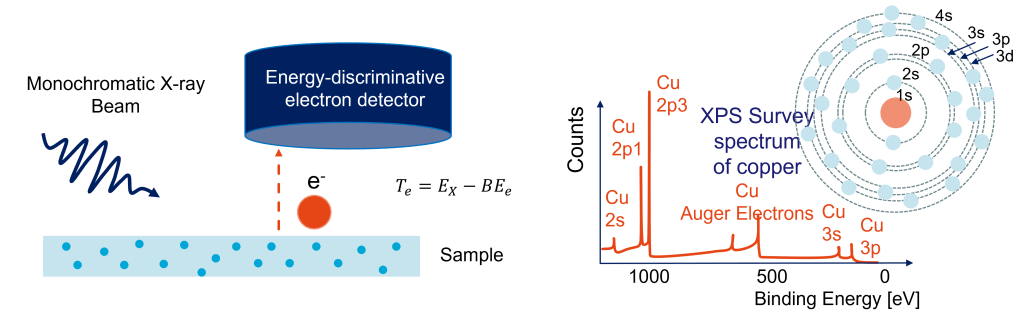
X-ray photoelectron spectroscopy (XPS) is an experimental technique that allows one to identify the presence and the valence/chemical bonding of elements on the top 2-10 nm of a sample. Combined with stepwise etching the sample, it can provide a depth-dependence of the quantity and chemical bonds of almost all elements. XPS relies on the photoelectric effect: it illuminates the sample with monochromatic X-rays. This illumination may cause the photoelectric effect to occur.
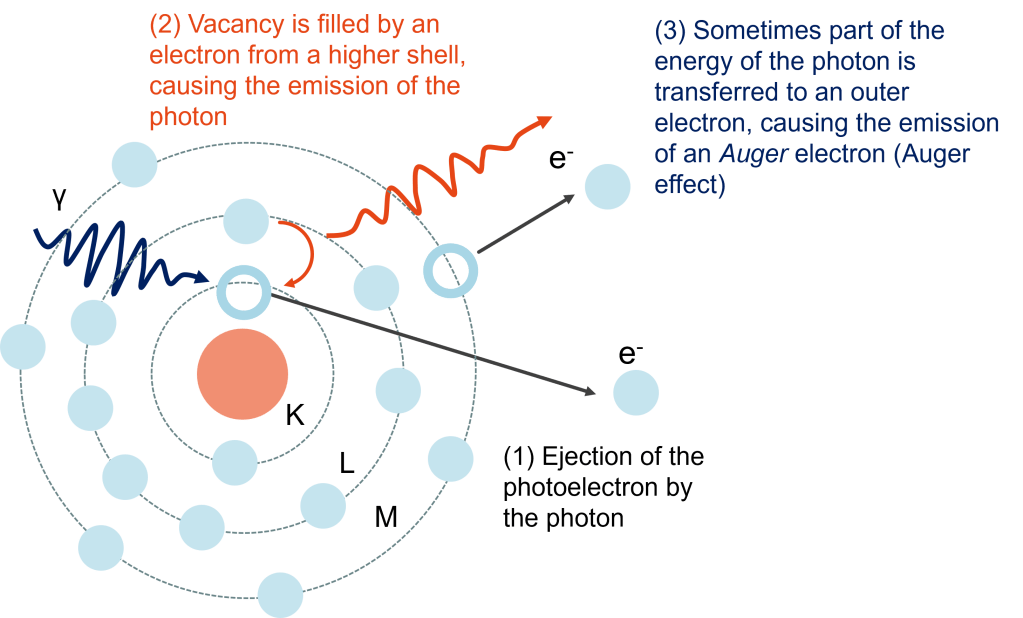
In the photoelectric effect, the X-ray is absorbed and an electron is liberated from the nucleus. By detecting this electron and determining its kinetic energy, one use the conservation of energy to determine the binding energy of the ejected photoelectron. The binding energies of the ejected photoelectrons, are unique to an element, allowing the identification (‘fingerprinting’) of the elements present in a material. On top of that, these binding energies are affected by the presence of chemical bonds or the valence of an atom.
For our research we often use the Thermofisher k-alpha X-ray photoelectron spectrometer of the Department of Chemical Engineering. It is equipped with a monochromatic Al X-ray source. A vacuum transfer box is used to transfer air-sensitive samples from a glove box to the spectrometer. In addition, it features an Ar-ion gun, allowing us to selectively stepwise etch samples to obtain depth profiles. More information about this machine can be found here: https://www.morechemistry.com/xps-info/agenda.html